ACS Research News
Raise Your Awareness About ACS Colorectal Cancer Research
Published on: March 5, 2024
March is Colorectal Cancer Awareness Month. See how American Cancer Society researchers are helping put an end to this disease.
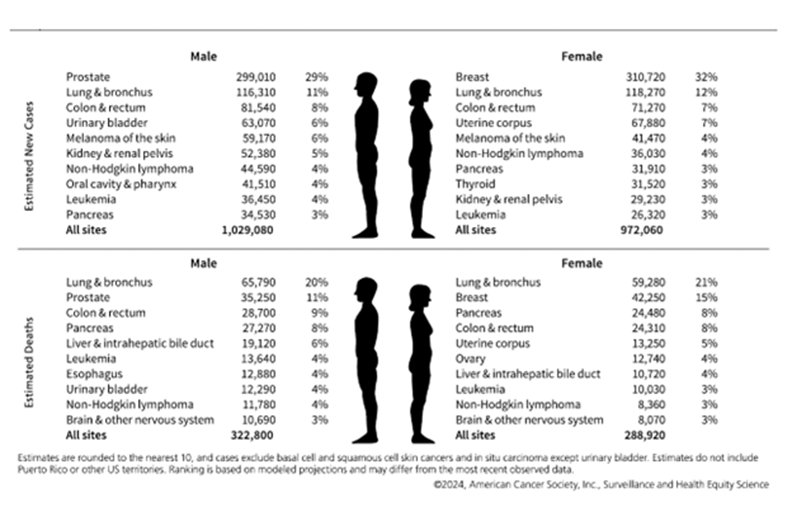
2024--First Year the US Expects More than 2M New Cases of Cancer
Published on: January 17, 2024
The American Cancer Society reports lower overall cancer death rates, yet incidence is increasing for many common cancers, including 6 of the top 10.
New Lung Cancer Screening Guideline Increases Eligibility
Published on: November 1, 2023
The updated ACS guideline recommends adults ages 50-80 who have a 20+ pack-year smoking history get screened with a low-dose CT scan each year.
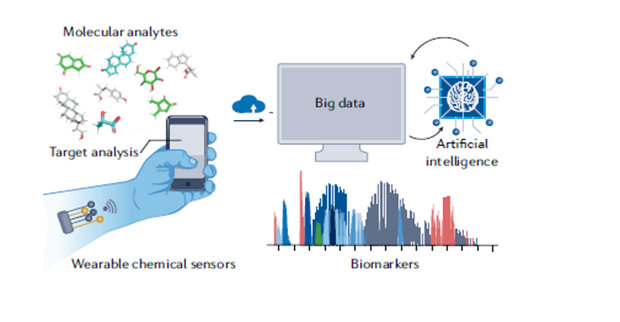
One Day, Prep for Stem Cell Transplants May Be at Home
Published on: March 30, 2023
ACS grantee, Wei Gao, PhD, tests a sweat sensor to see if it quickly IDs a personalized dose for 2 chemotherapy drugs that AML patients receive before a stem cell transplant.